
- ATOMIC EMISSION SPECTRUM VS CONTINUOUS SPECTRUM HOW TO
- ATOMIC EMISSION SPECTRUM VS CONTINUOUS SPECTRUM SERIES
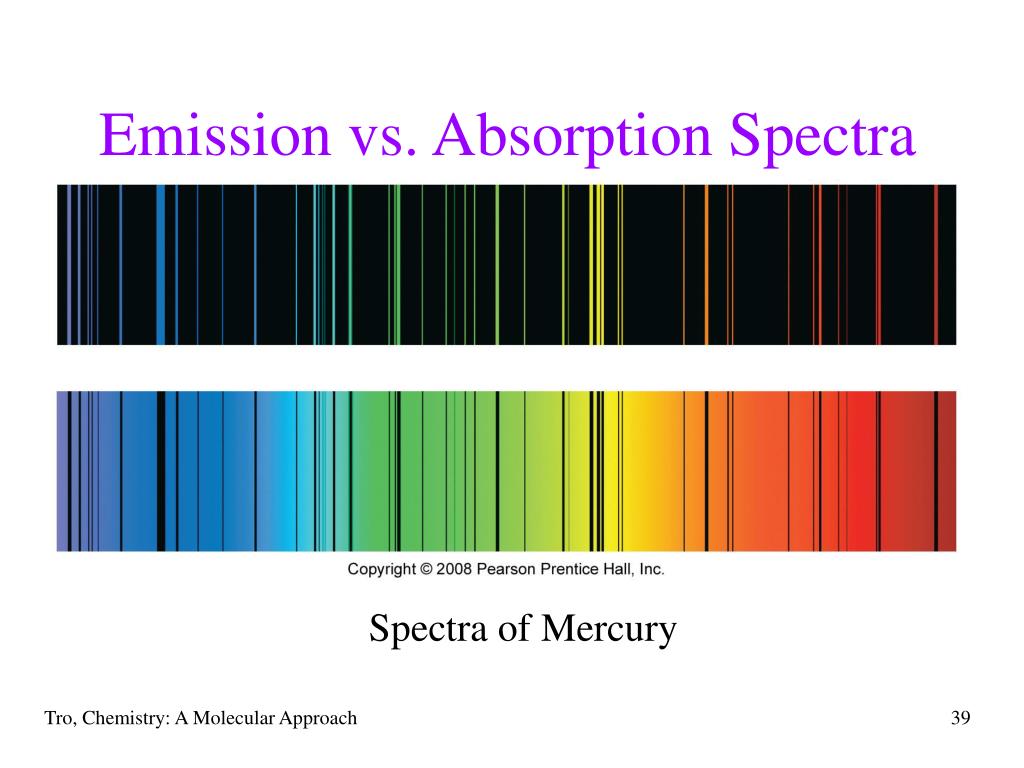
Recall that the energy carried by a photon is given by E = h ν This equation is not rendering properly due to an incompatible browser. In the lower panel, the electrons are absorbing photons, causing them to jump to higher levels from their lower levels. In the top panel, the electrons are falling from higher levels to lower levels and are emitting photons.
ATOMIC EMISSION SPECTRUM VS CONTINUOUS SPECTRUM SERIES
Once an electron is in a higher level, it will eventually fall back down to a lower level (either all at once back down to level 1, or by a series of steps down to level 1), and each time it falls from one level to a lower one, it emits a photon that carries exactly the amount of energy equal to the difference in energy between the starting energy level and the ending energy level of the electron. If an electron absorbs exactly the energy difference between the level it is in and any higher level, it can move up to a higher level. Thus, the orbits can also be referred to as energy levels. Each orbit has a specific energy associated with it-that is, when the electron is in a specific orbit, it has a specific amount of energy. The crucial part of his model is to understand that the electrons can only exist in these specific orbits, and not in between. Bohr proposed a simple model for atoms that required the electrons to occupy “orbits” around the nucleus. Returning to atomic physics and spectroscopy, it is the electrons that are the primary cause of the absorption lines we see in stellar spectra. Here is a cartoon image I put together of a helium atom:įor a bit more background and information on models of the atom, see a description by the folks at the Jefferson Lab. Surrounding the nucleus are one or more negatively charged electrons. The atoms inside the cloud of gas are made up of a nucleus of positively charged protons and neutrons, which have no charge. A typical cloud of gas in space is likely to contain a lot of hydrogen and helium and trace amounts of heavier elements, like oxygen, nitrogen, carbon, and perhaps iron.
:max_bytes(150000):strip_icc()/GettyImages-1096547948-35b3799817ca4b2fa06888893ef4a348.jpg)
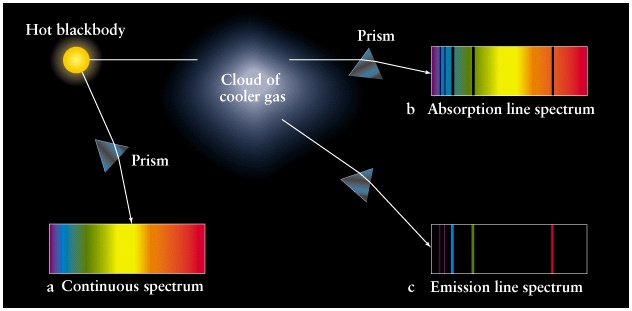
So, what is going on inside the gas?Ī cloud of gas is made up of atoms, which are the smallest components of an element that retain all the properties of that element. Somehow, it is the gas that causes the absorption lines to appear in what would otherwise appear to be a continuous spectrum. We observe absorption lines when the light from a background source passes through a cool gas.
ATOMIC EMISSION SPECTRUM VS CONTINUOUS SPECTRUM HOW TO
The differences in these spectra and a description of how to create them were summarized in Kirchhoff’s three laws of spectroscopy: In the early days of spectroscopy, experiments revealed that there were three main types of spectra. Other astronomical sources (and also light sources you can test in a lab) are found to create spectra that show little intensity at most wavelengths but a few precise wavelengths where a lot of intensity is seen. These gaps in the spectrum where there is no light emitted are called absorption lines. If you look at the two spectra of stars, you see there are black bands in the image of the sun’s spectrum and areas in the plot where the intensity goes to zero or nearly zero in the spectrum of the blue straggler. Recall that blackbody radiation is continuous with no breaks. For example, consider the two spectra you looked at on a previous page: the sun and a blue straggler star. In reality, few objects emit exactly a blackbody spectrum.
However, I have stressed a few times that blackbody radiation is only emitted by an “ideal” or “perfect” radiator. Studying blackbody radiation is a useful exercise.


 0 kommentar(er)
0 kommentar(er)
